Starting on July 1 2016 Malaysias Medical Device Act has made it mandatory for all foreign manufacturers to register their medical device with the MDA. Facilitate trade and industry.

Children With Disabilities In Malaysia The Big Picture What To Write About Writing Classes Unicef
For the purpose of marketing.

. Medical Device Authority MDA is a statutory body under the Ministry of Health Malaysia which was established under the Medical Device Authority Act 2012 Act 738 to control regulate medical device its industry and activities as well as to enforce medical device law under Medical Device Act 2012 Act 737. Under the Medical Device Act 2012 the manufacturer or the Local Authorized Representative of the foreign manufacturer is required to register a medical device before importing exporting or placing it in the Malaysia market. FIRST ANNOUNCEMENT - SEMINAR BY THE MEDICAL DEVICE AUTHORITY ON.
The Medical Device Regulations 2012 the subsidiary legislations under the Medical Device Act 2012 Act 737 has been approved by the Malaysian Minister of Health and has been published in the Gazette on 31st December 2012. Or b private hospital private medical clinic dental clinic or healthcare. In Malaysia the medical device industry is a highly diversified industry that produces a broad range of products and equipment ranging from medical gloves implantable devices orthopaedic devices and.
License and Permit Part IV. The gazettement took effect on 3rd September 2019. Additionally all foreign manufacturers must now obtain Conformity Assessment Body CAB certification in order to receive MDA approval for.
As to that effect the Medical Device Authority encourages. 27th September1971 Date of publication in the Gazette. Enacted by the Parliament of Malaysia as follows.
There is a wide range of medical devices from a simple medical device to a highly complex and sophisticated medical device. Government Authority Medical device product registration in Malaysia is overseen by the Medical Device Authority MDA of the Ministry of Health Malaysia MoHM as stipulated under the Medical Device Act 2012 which was made effective on July 1 2013. Official Portal of Medical Device Authority MDA Malaysia.
Exemption from registration of medical devices. HOW TO APPLY FOR MEDICAL DEVICE REGISTRATION UNDER ACT 737 MDAGL No 1. Medical Device Exemption Order 2016 gazetted on April 18 2016.
PERCETAKAN NASIONAL MALAYSIA BHD 2006 Act 50 MEDICAL ACT 1971 Incorporating all amendments up to 1 January 2006 050e A132 of 02 A93-03 A177-03 A391-03 PUA11604fm Page 1 Friday March 31 2006 1049 AM. All were published by the Medical Device Control Division Ministry of Health Malaysia MDCD. Recent Medical Device Authority MDA policies target five key areas.
Vision Mission Core Value. Malaysia Medical Devices Regulations. FOR THE PURPOSE OF MEDICAL DEVICE REGISTRATION UNDER THE ACT 737 - 29.
Authorized representation Conformity Assessments registration exemptions exportimport requirements and Good. The Malaysian Government gazetted the Medical Device Advertising Regulations 2019 Advertising Regulations and the Medical Device Duties and Obligations of Establishments Regulations. Pa rt i PRELiMiNARY short title and commencement 1.
The Regulations will come into operation simultaneously with Act 737 on 1st July 2013. Registration Of Medical Device And Conformity Assessment Body Part III. MINISTRY OF HEALTH MALAYSIA.
According to the act medical devices require registration with the MDA before being imported and placed on the market. Registration of medical device is granted for five years. PREPARED FOR PUBLICATION BY MALAYAN LAW JOURNAL SDN BHD AND PRINTED BY.
Citation and commencement 1. Medical Device Act Act 737 2012 Medical Device Authority Act Act 738 2012 Passed by Lower House of Parliament. These Regulations may be cited as the Medical Device Regulations 2012 and shall come.
However the Regulations will only come into operation on 1st July 2020 to allow stakeholders adequate time to comply with all the requirements. Medical Device 7 laws OF MalaYsIa act 737 MedIcal devIce act 2012 An Act to regulate medical devices the industry and to provide for matters connected thereto. July 2013 1 Introduction 1 Section 51 of Medical Device Act 2012 Act 737 requires a medical device to be registered under the Act before it can be imported exported or placed in the market.
Malaysia Medical Device Regulations. 11 rows Medical Device Act 2012. All advertisements that promote the medical device to general public needs to attain approval from MDA through email.
1 This Act may be cited as the Medical Device Act 2012. Medical Device Act 2012. For the purpose of personal use 2.
This is the latest gazettement of the Medical Device Regulations pursuant to the Medical Device Act 2012 Act 737. Medical Device Exemption Order 2016. The minister exempts any medical device from section 5 of the Act Act 737 if the medical device is.
Yes medical devices do require registration before they can be sold in Malaysia. 30 September 1971 Date of coming into operation. Malaysia MDA Announcement Updated.
Medical device market regulators in Malaysia have implemented several new policies pertaining to the countrys recently enacted Medical Device Act. In exercise of the powers conferred by Section 79 of the Medical Device Act 2012 the Minister of Health hereby makes the following Regulations. Our Hotline 603 - 8230 0300.
The guidance document explains among others what information a label should contain what kind of format is allowed and the location of labelling. 1 October 1971 _____ ARRANGEMENT OF SECTIONS _____ ACT 50 MEDICAL ACT 1971 PART I - PRELIMINARY Section 1. BACKGROUND MEDICAL DEVICE AUTHORITY Ministry of Health Malaysia Level 5 Menara Prisma Boulevard Plot 3C4 No 26 Persiaran Perdana Precinct 3 62675 Putrajaya MALAYSIA ˇ.
LAWS OF MALAYSIA ACT 50 MEDICAL ACT 1971 Incorporating latest amendment - PUA 172 2005 Date of Royal Assent. The Medical Device Authority MDA has prepared a guidance document on labelling requirements for medical devices under the Medical Device Act Act 737 and its regulations. Jun 12 2014.
Pihak Berkuasa Peranti Perubatan KEMENTERIAN KESIHATAN MALAYSIA Background Medical Devices Regulatory System Medical Device Authority Act 2012 Act 738 Medical Device Act 2012 Act 737 Part I Preliminary Part II.

General Medical Device Medical Device Authority Mda

Browse Our Sample Of Dividend Payment Voucher Template Dividend Templates Voucher

Flow Chart The Official Portal Of Intellectual Property Corporation Of Malaysia Flow Chart Patent Registration Trips Agreement

Company Super Form Malaysia Malaysia Company Make Business

Top 5 Environmental Stats Industrial Water Pollution Infographic Intelex Water Waterquality Waterpollution Pol Water Pollution Pollution Infographic

Medical Device Registration In Malaysia

Human Rights And The Rule Of Law Rule Of Law Institute Of Australia Master Of Laws Human Rights Cyber Warfare
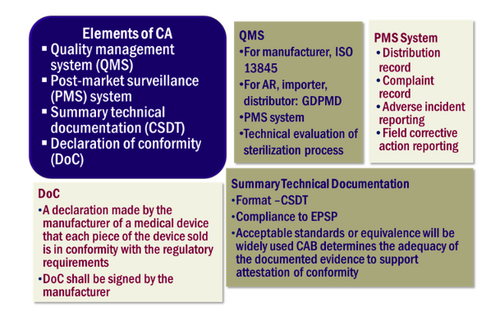
General Medical Device Medical Device Authority Mda
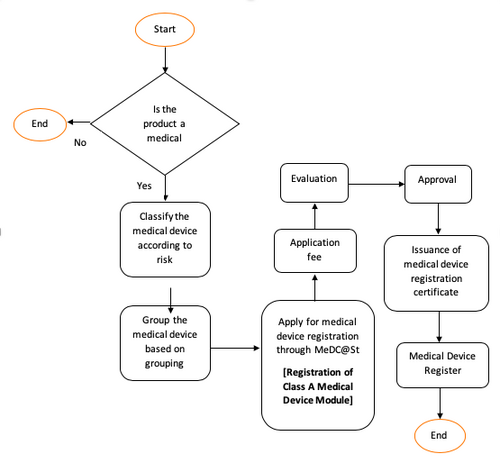
General Medical Device Medical Device Authority Mda

1 In 5 Americans Track Their Health Statistics Using An App Marketing Charts Health Statistics Health App Healthcare Ads
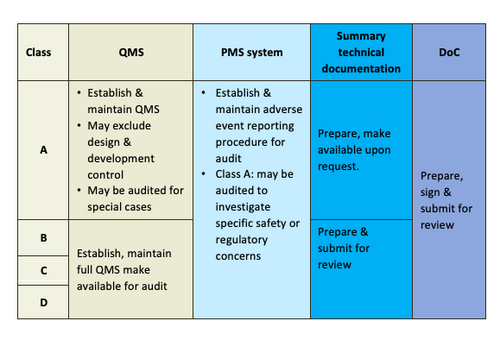
General Medical Device Medical Device Authority Mda

Private Varsity Opens Medical Centre Management And Science University Medical Top Universities
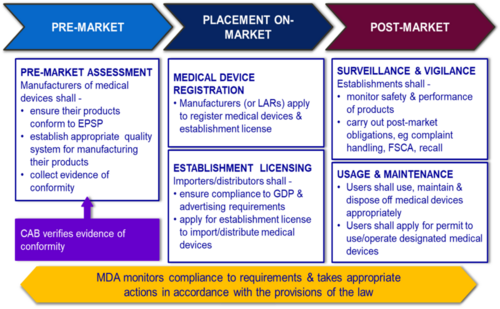
General Medical Device Medical Device Authority Mda
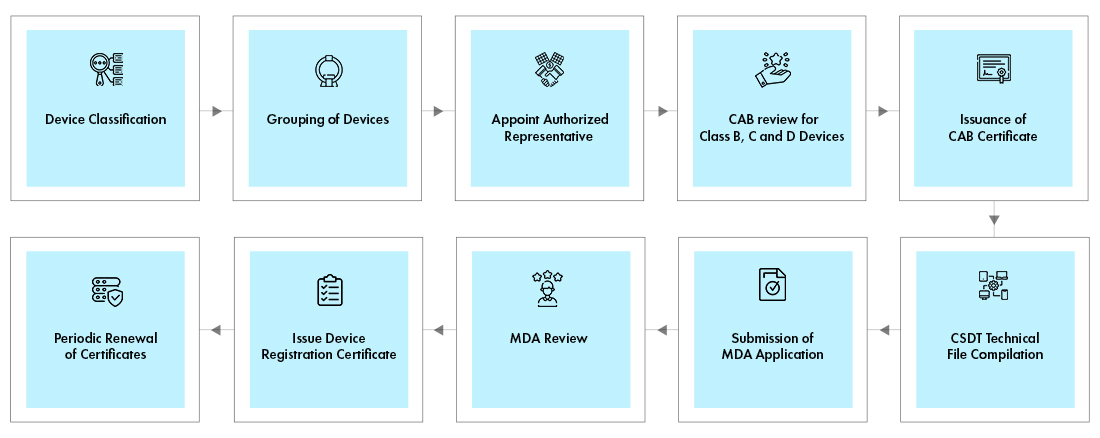
Medical Device Registration Malaysia Mda Malaysia Medical Device Classification
Malaysia Mda Updates Medical Device Labelling And Change Notification Guidance Documents Thema Med
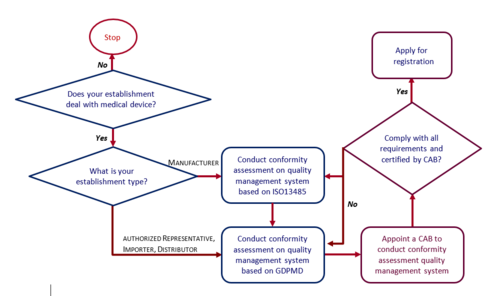
How To Apply For Establishment Licence Medical Device Authority Mda

Pin By Khoo Kimeng On Message S Frosted Flakes Cereal Box Cereal Box Frosted Flakes

Free Temporary Guardianship Form Template Inspirational Sample Temporary Guardianship Form 9 Download Documen Guardianship Printable Letter Templates Lettering

